Brief introduction of Denitration Process

NO
Xcontrol method starts from three stages of the fuel life cycle, namely before combustion, during combustion and after combustion. At present, there are few researches on denitrification before combustion, and almost all descriptions focus on the control of NO
X during combustion and after combustion. Therefore, in the world, all the NO
X control measures in combustion are collectively referred to as primary measures, and the
NOXcontrol measures after combustion are collectively referred to as secondary measures, also known as flue gas denitrification technology.
At present, the commonly used NOX control technology in combustion is low NOX combustion technology, mainly including low NOX burner, air staged combustion and fuel staged combustion.
The mature flue gas denitration technologies applied in coal-fired power plant boilers mainly include:
1、 Process principle of selective catalytic reduction (SCR)(Selective Catalytic Reduction,(SCR)
1) SCR Process principle:
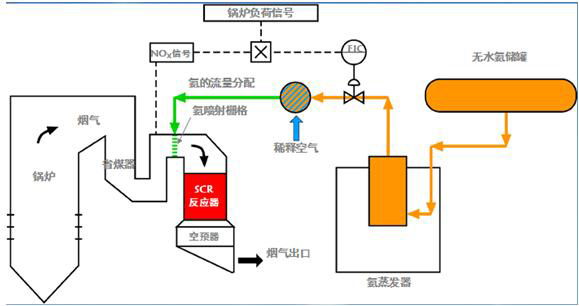
2) Reaction Principle:
The basic principle of selective catalytic reduction (SCR) technology is to reduce nitrogen oxide (NOx) in flue gas to nitrogen and water by injecting ammonia (NH3) into flue gas as reducing agent under the action of catalyst. The denitration efficiency of this technology can reach 90-95%, and the emission concentration of NOx can be completely controlled below 100mg / Nm3.
The reaction process in the catalyst is mainly as follows:
4NO +4NH3 + O2 → 4N2 + 6H2O
6NO2 + 8NH3 → 7N2 + 12H2O
2、(Selective Non-Catalytic Reduction,简称SNCR)
1) SNCR

2)Reaction principle
Selective non catalytic reduction is a selective reaction of NOx with reacting agents like NH3, urea etc. injected into the furnace, based on which SNCR method is developed. It is found that in the narrow temperature range of 850-1150 ℃ in the furnace and without catalyst, ammonia reducing agents such as NH3 or urea can selectively reduce NOx in the flue gas. The reducing agent (urea) rapidly decomposes into NH3 and reacts with NOx in the flue gas by SNCR to generate N2. This method takes the furnace as the reactor and basically does not interact with O2 in the flue gas. In the range of 850-1150 ℃, the main reactions of NOx reduction by NH3 or urea are as follows:
When NH3 is reducing agent:
4 NH3 + 4NO +O2 →4N2 + 6H2O
When urea as reducing agent:
2CO(NH2)2+O2→4N2+2CO22O







